
We develop and promote complex systems like medical products, scientific concepts and software solutions.
Medical Software and Apps according to ISO13485 Standard

Stunning
mobile apps
Native and cross platform
iOS and Android development

Complex and robust
cloud systems
Reliable, performant and secure according to the latest standards

Beautiful data driven dashboards
On the web, smartphone
or on your tablet

ISO13485
certified
Quality Management System Medical Devices
Why choose us?
Coming from the medical sector where highest quality standards and clear strategies to verify and validate the development outcome are essential, we managed to transfer this knowledge to other sectors resulting in the desired results.
Our expertise combines a strong scientific background, solid engineering and quality management capabilities, practical knowledge in various regulatory pathways with vast experience in business development.
Therefore, we see ourselves as optimal partner to realize complex and demanding projects and to transfer great innovations to commercial successes.
Certificates

TÜV SÜD
ISO 13485
Quality Management System
Medical Devices
Class II Product Approval
according to EU Medical Device
Regulation 2017/745

US FDA Clearance
according to 510(k)

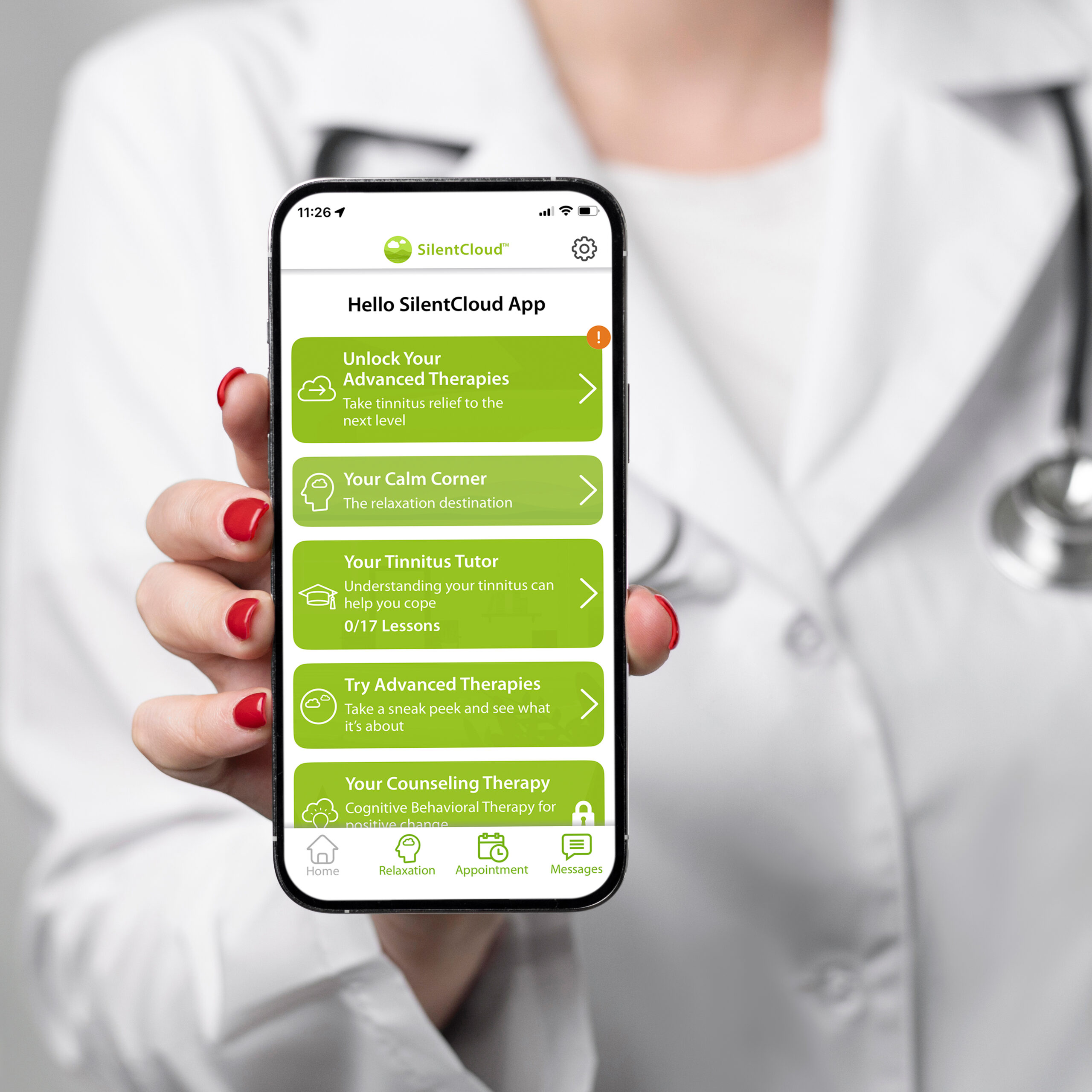
SilentCloud™ Tinnitus Management App
Contact
Aureliym GmbH
Hauptstraße 96
53474 Bad Neuenahr-Ahrweiler
Germany